
PULSE
Predictive AI for Cardiac Monitoring

PULSE
Predictive AI for Cardiac Monitoring

Predictive AI for Cardiac Monitoring

Predictive AI for Cardiac Monitoring
Cardiac arrest is a leading cause of morbidity and mortality. Each year in the U.S. alone, it affects approximately 300,000 adults in hospital and 250,000 out of hospital, with an average patient age of 66 years. In-hospital cardiac arrest represents a true clinical emergency requiring immediate intervention to improve the chances of survival and intact neurologic functioning. Yet, only about 25% of patients survive to discharge.
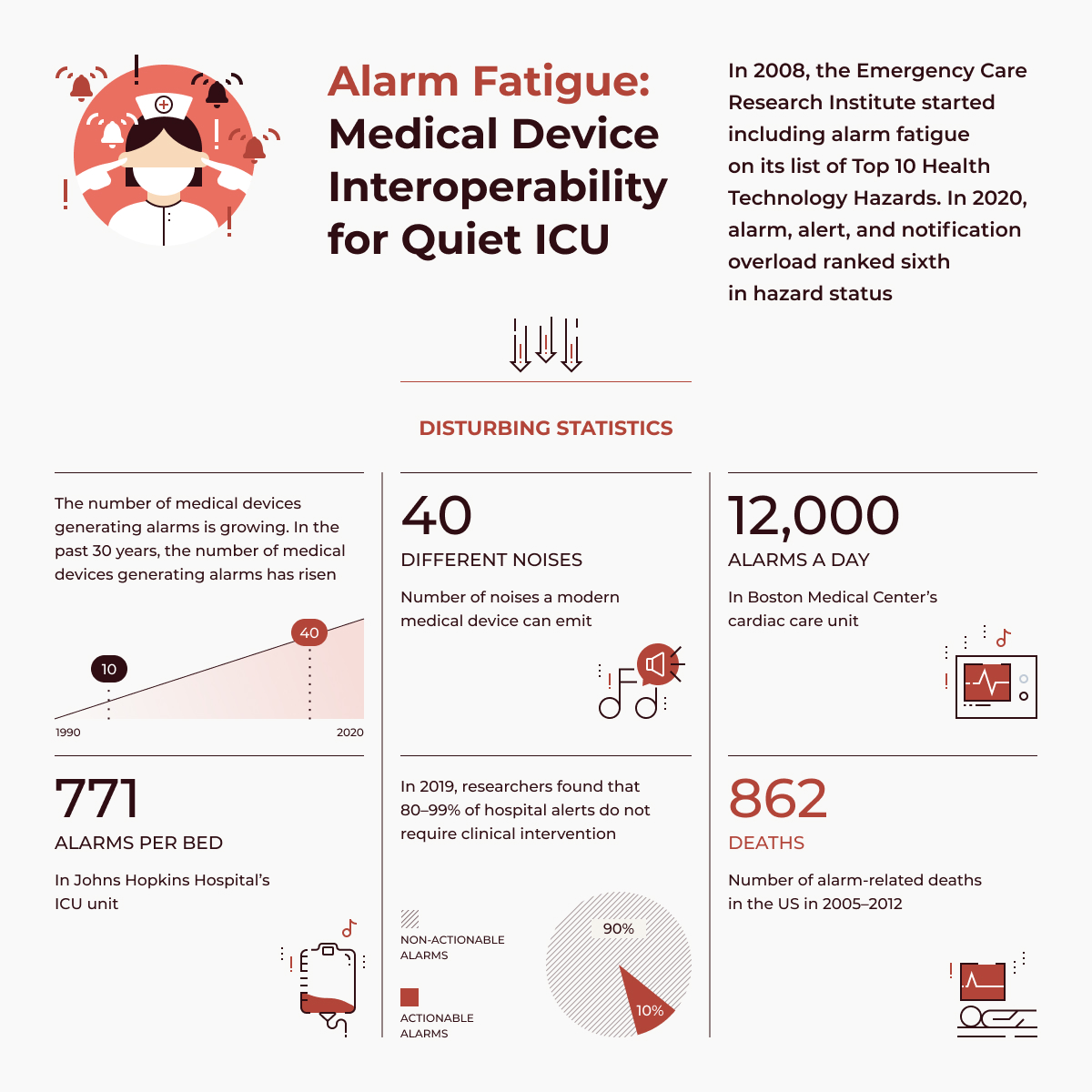
Retrospective studies indicate that poor clinical monitoring is a primary cause of preventable deaths. Current monitoring systems in the ICU are unable to identify impending cardiac arrests despite post-hoc analysis by cardiologists revealing high-risk signs. Moreover, they suffer from false alarm rates of over 85%, leading to alarm fatigue.
PULSE is a cross-disciplinary initiative that aims to address this gap by combining advanced AI modeling with high-resolution, real-time, ECG data from patients admitted to the ICU. Our goal is to develop AI-driven models that provide timely, clinically actionable predictions while substantially reducing false alarms.
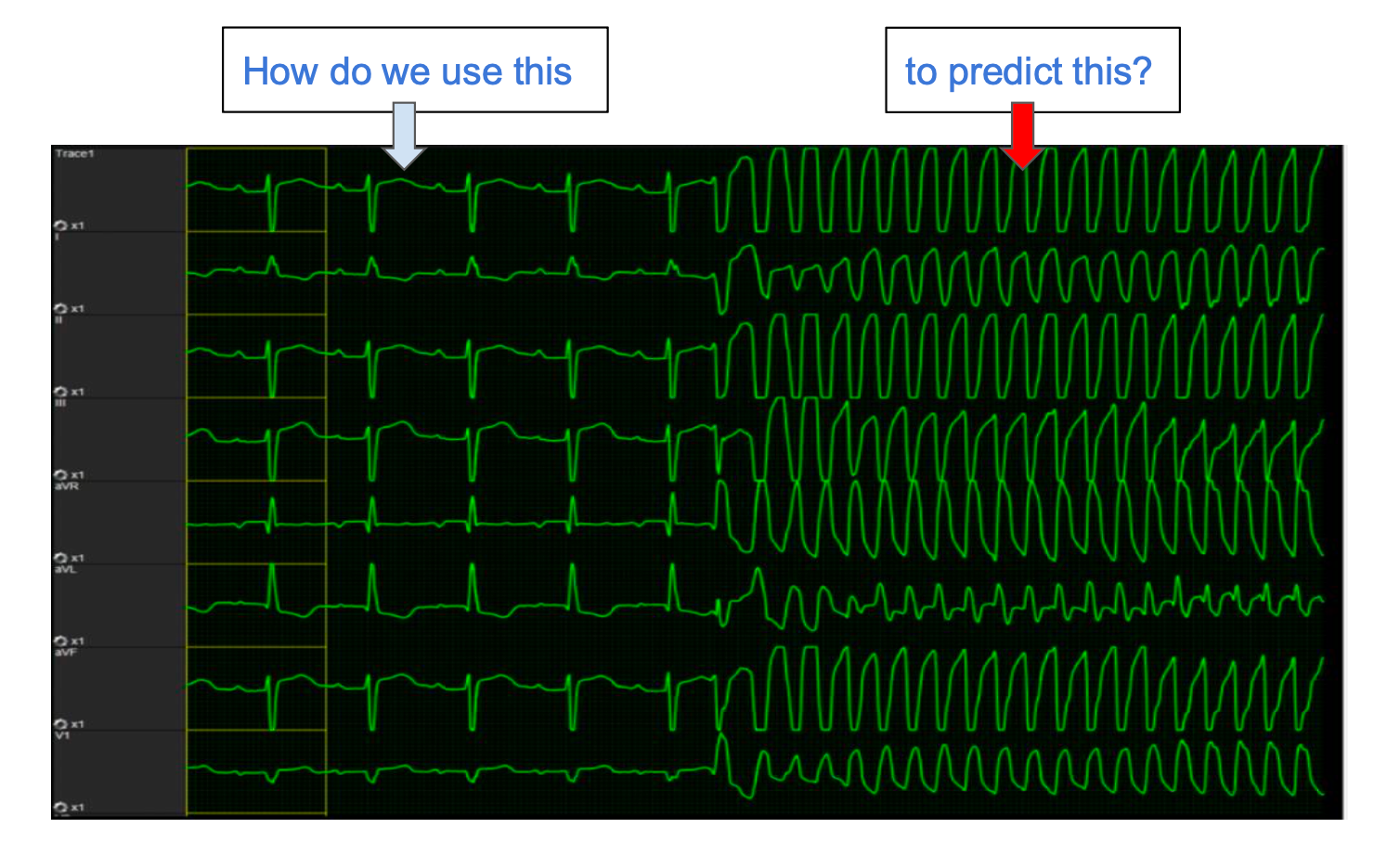
The core challenge in developing prognostic models relates to the ability to distinguish between clinically informative ECG patterns and noise over long-horizon data. Our target is less than 1 false alarm per 3 hours per hospitalized patient and to identify impending cardiac arrest minutes to hours prior to the event.
The potential of successfully deploying such models is very high:
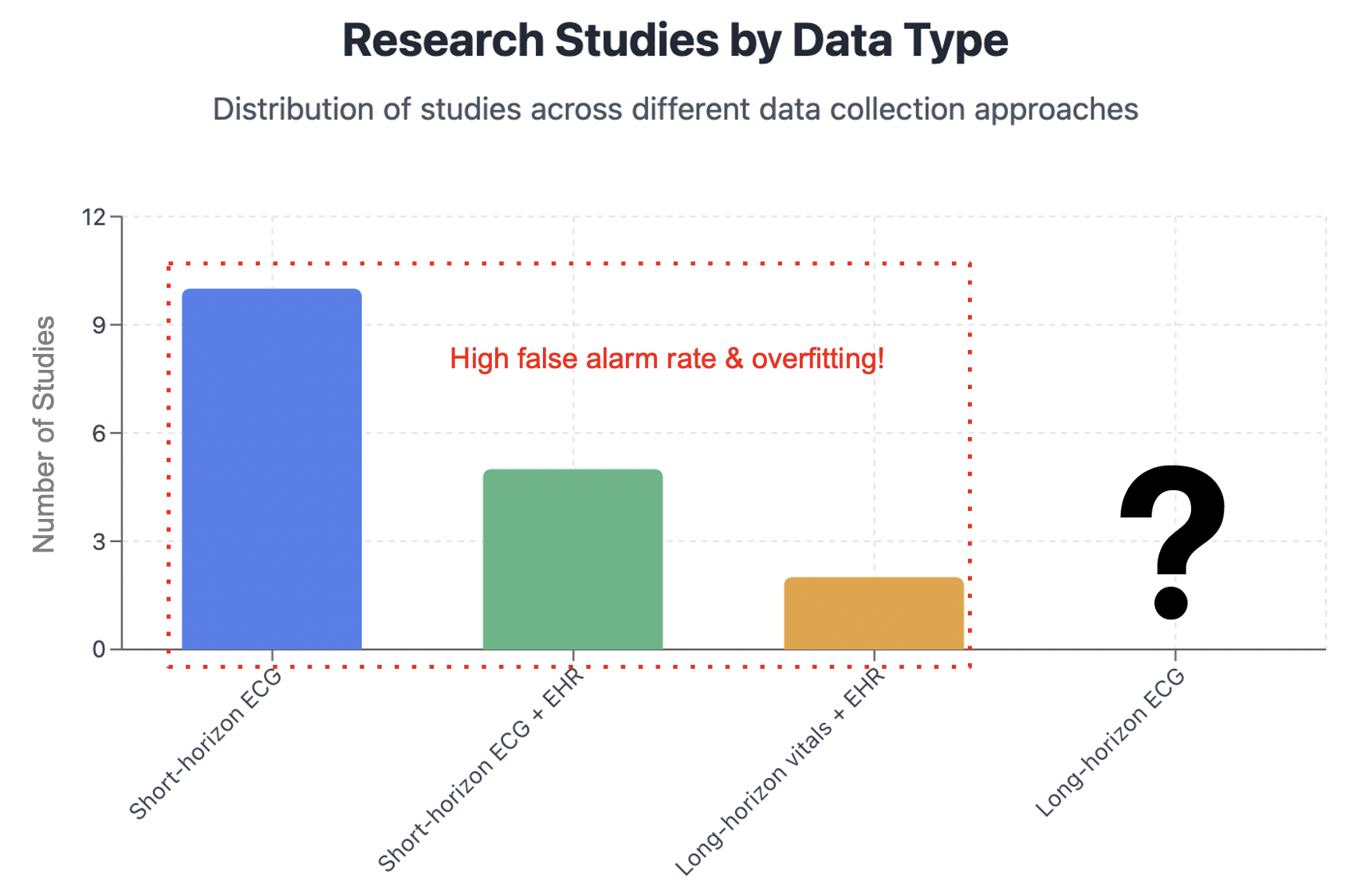
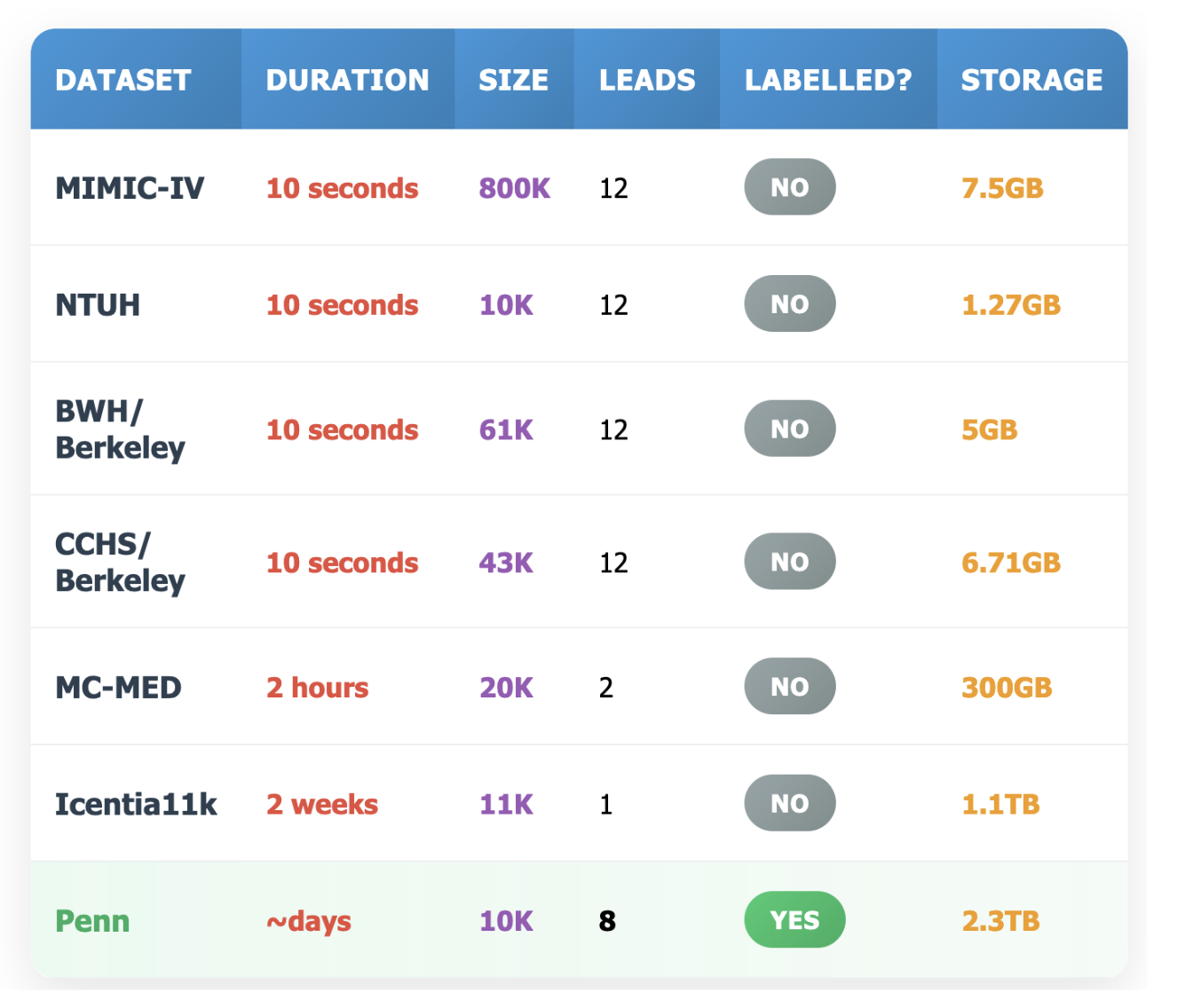
⚠️Insufficient Data Quality The quality of data is central to the success of any AI solution. In contrast to domains like code generation or protein folding—where AI has thrived thanks to abundant high-quality data—public ECG datasets typically contain only 10-second or single-lead recordings. These are insufficient to train generative models or to predict cardiac arrest with adequate lead time.
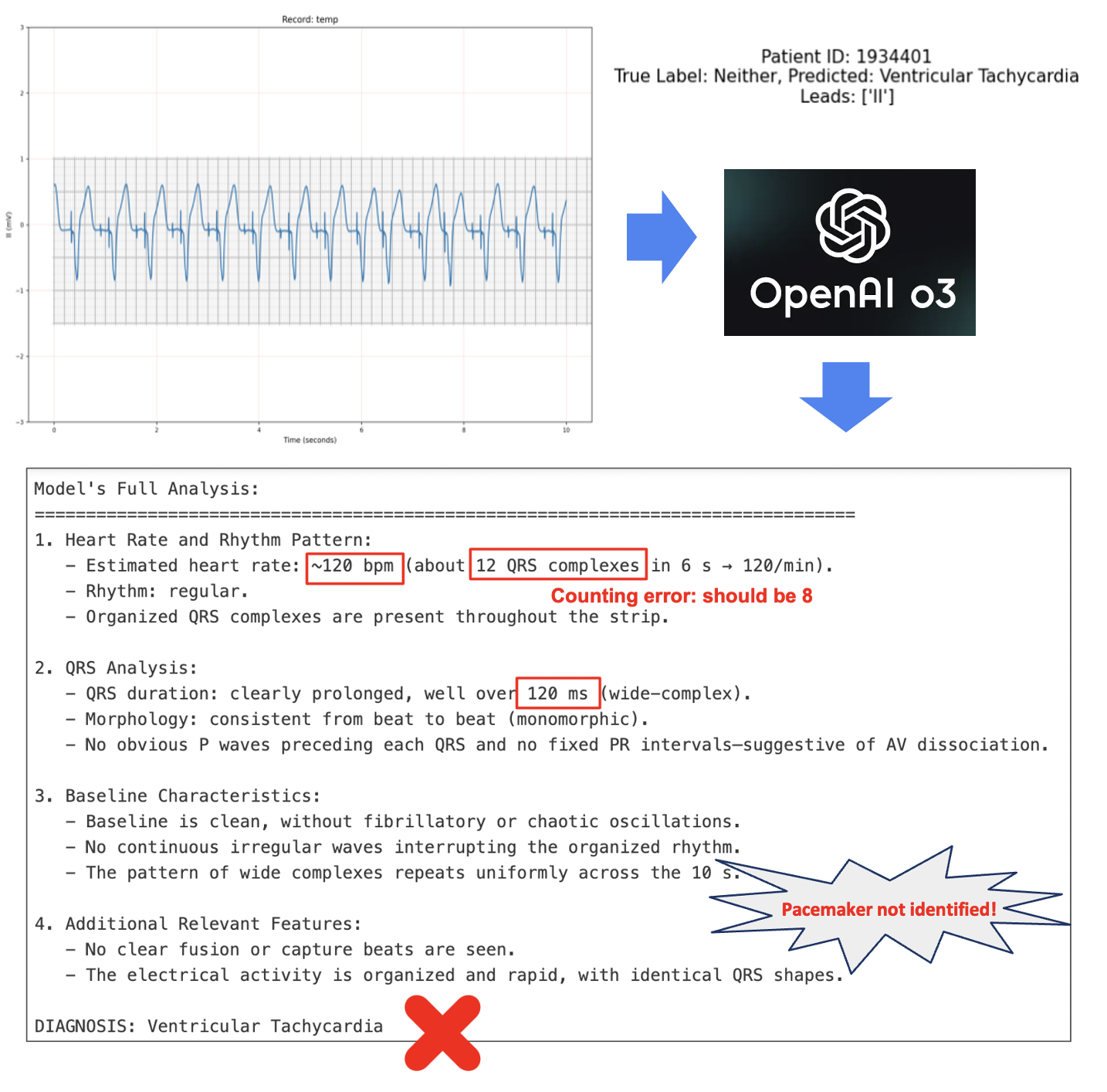
⚠️Low Accuracy and Hallucinations When trained on such limited data, AI models fall short of clinical utility. For instance, a deep learning model published in early 2025 reported a specificity of just 31% when sensitivity was fixed at 95%—a false positive rate of 69%. Compounding the issue, prompting state-of-the-art deep learning models like OpenAI's o3 directly on these datasets can lead to hallucinations and unreliable outputs.
⚠️Lack of Clinical Usefulness As a result, many AI systems in this space fail to translate into meaningful clinical applications. The disconnect between technical development and real-world clinical considerations—such as alarm fatigue or workflow integration—prevents these models from being deployed effectively in the ICU.
Our approach comprises three fundamental pillars: (1) generating the largest high-quality long-horizon ECG dataset; (2) building accurate and explainable predictive AI models; and (3) translational integration and clinical validation.
✓High-Quality Long-Horizon ECG Dataset We will leverage novel, high-resolution, long-horizon ECG data at Penn Medicine, collected from monitoring 10,000 patients. These data, sampled at 250Hz, will provide the crucial fine-grained information that are unavailable in any public dataset.
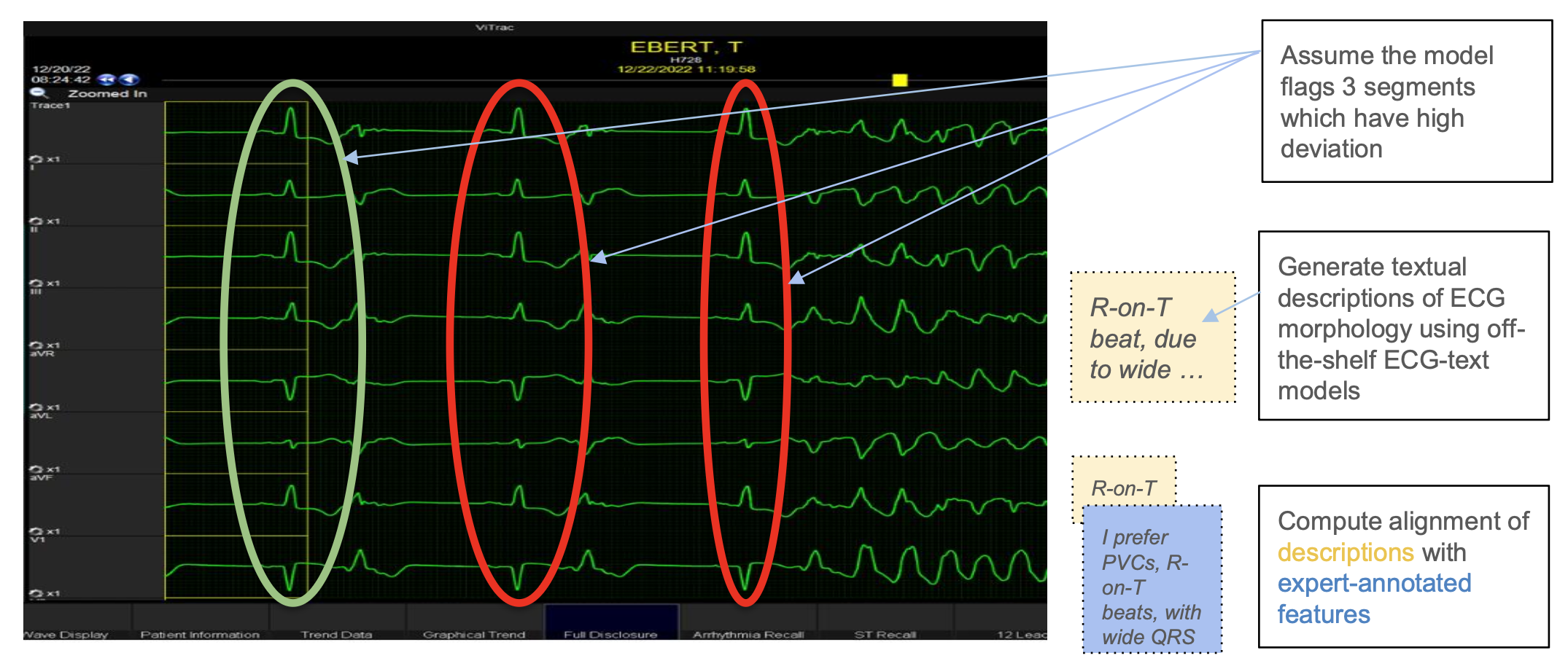
✓Accurate and Explainable Predictive AI Models We have unique expertise in neurosymbolic AI—an approach that blends deep learning's ability to detect complex physiological patterns with symbolic reasoning that provides guardrails by grounding predictions in clinical reality. Their work is rooted in mathematical rigor, systems engineering, and state-of-the-art methods for reliability and interpretability—making them uniquely equipped to build models that are not only accurate but also deployable in high-stakes settings.
✓Translational Integration and Clinical Validation Our clinical colleagues have extensive expertise in developing and validating prognostic models for sudden cardiac death and fatal cardiovascular disease. Consequently, this collaboration is deeply integrated across both domains—from acquiring and interpreting raw telemetry data to designing models that support clinical decision-making. Our designs are modularly integrated into platforms for clinical evaluation. This initiative will include a shared computational platform for generative design and automated experimental facilities for high-throughput analysis, with the ultimate goal of translating our findings directly into improved patient outcomes.











